NFPA Chemicals
NFPA 704 Marking System
The National Fire Protection Association (NFPA), in section 704 of the National Fire Code, specifies a system for identifying the hazards associated with materials. Information contained on this and linked pages comes directly from the 1990 edition of NFPA 704. Although the system was developed primarily with the needs of fire protection agencies in mind, it is of value to anyone who needs to handle potentially hazardous material.
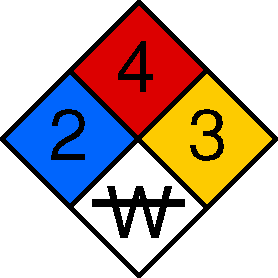
Blue | Red | Yellow | White
The NFPA 704 Standard System for the Identification of the Hazards of Materials for Emergency Response, was developed as a manual by the Sectional Committee on Classification, Labeling and Properties of Flammable Liquids of the NFPA Committee on Flammable Liquids starting in 1952. Its first adoption as a guide occurred in 1961, with regular revisions being adopted through 1987. In 1990 it became an NFPA standard, and extensive quantitative health hazard rating criteria were introduced. The purpose of the standard, as originally conceived, is to safeguard the lives of those individuals who respond to emergencies occuring in an industrial plant or storage location, or other location where relatively large quantities of chemicals are used, and where the hazards of materials are not readily apparent.
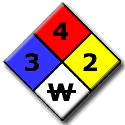
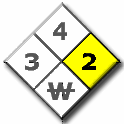
The hazard identification signal is a color-coded array of four numbers or letters arranged in a diamond shape. An example is shown to the right. You will see hazard diamonds like this on trucks, storage tanks, bottles of chemicals, and in various other places around campus and around town.
The blue, red, and yellow fields (health, flammability, and reactivity) all use a numbering scale ranging from 0 to 4. A value of zero means that the material poses essentially no hazard; a rating of four indicates extreme danger. The fourth value (associated with white) tends to be more variable, both in meaning and in what letters or numbers are written there.
RelatedProducts
NFPA 704 Ratings for Common Chemicals
This page is for general reference and educational purposes only and should NOT be used to determine regulatory compliance or relied upon as a sole source of information where matters of life and health are concerned. This site and the author do not warrant or guarantee the accuracy or the sufficiency of the information provided and do not assume any responsibility for its use.
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z |
| NFPA 704 RATINGS FOR COMMON CHEMICALS | ||||
| Chemical | Health | Fire | Reactivity | Special |
| Acetaldehyde | 2 | 4 | 2 | |
| Acetic acid (glacial) | 2 | 2 | 0 | |
| Acetic anhydride | 2 | 2 | 1 | |
| Acetone | 2 | 3 | 0 | |
| Acetone cyanohydrin | 3 | 2 | 2 | |
| Acetonitrile | 3 | 3 | 0 | |
| Acetophenone | 1 | 2 | 0 | |
| Acetyl chloride | 3 | 3 | 2 | w |
| Acetylene | 1 | 4 | 3 | |
| Acrolein, inhibited | 3 | 3 | 3 | |
| Acrylamide | 3 | 2 | 2 | |
| Acrylic acid (glacial) | 3 | 2 | 2 | |
| Acrylonitrile | 4 | 3 | 2 | |
| Adiponitrile | 4 | 2 | 1 | |
| Alkylaluminums | 3 | 4 | 3 | w |
| Ally Chlorocarbonate | 3 | 3 | 1 | |
| Ally alcohol | 3 | 3 | 1 | |
| Ally bromide | 3 | 3 | 1 | |
| Ally chloride | 3 | 3 | 1 | |
| Allylamine | 3 | 3 | 1 | |
| Aluminum | 0 | 1 | 1 | |
| Aluminum Chloride | 3 | 0 | 2 | w |
| Aluminum Phosphide | 3 | 4 | 2 | w |
| Ammonia (anhydrous) | 3 | 1 | 0 | |
| Ammonium Dichromate | 2 | 1 | 1 | ox |
| Ammonium Fluoride | 3 | 0 | 0 | |
| Ammonium Nitrate | 1 | 0 | 3 | ox |
| Ammonium Perchlorate | 1 | 0 | 4 | ox |
| Ammonium Permanganate | 1 | 0 | 3 | ox |
| Amyl Mercaptan | 2 | 3 | 0 | |
| Amyl Nitrate | 2 | 2 | 0 | |
| Amylamine | 2 | 3 | 0 | |
| Aniline | 3 | 2 | 0 | |
| Antimony pentachloride | 3 | 0 | 1 | |
| Antimony Pentafluoride | 3 | 0 | 1 | |
| Antimony Sulfide, solid | 2 | 1 | 1 | |
| Arsenic Pentafluoride | 4 | 0 | 1 | |
| Arsenic Pentoxide | 2 | 0 | 0 | |
| Arsenic Trichloride | 3 | 0 | 0 | |
| Arsenic Trioxide | 2 | 0 | 0 | |
| Arsenic Trisulfide | 2 | 0 | 0 | |
| Arsine | 4 | 4 | 2 | |
| Chemical | Health | Fire | Reactivity | Special |
| Barium Chlorate | 2 | 0 | 1 | ox |
| Benzaldehyde | 2 | 2 | 0 | |
| Benzene | 2 | 3 | 0 | |
| Benzotrifluoride | 3 | 3 | 1 | |
| Benzoyl Chloride | 3 | 2 | 2 | w |
| Beryllium Metal | 3 | 1 | 0 | |
| Boron Tribromide | 3 | 0 | 2 | w |
| Boron Trifluoride | 3 | 0 | 1 | |
| Bromine | 3 | 0 | 0 | ox |
| Bromine Pentafluoride | 4 | 0 | 3 | |
| Bromine Trifluoride | 4 | 0 | 3 | |
| Bromopentane, 1- | 1 | 3 | 0 | |
| Bromopropyne, 3- | 4 | 3 | 4 | |
| Butadiene, inhibited | 2 | 4 | 2 | |
| Butanol, 1- | 1 | 3 | 0 | |
| Butanol, 2- | 1 | 3 | 0 | |
| Butyl Acrylate, inhibited | 2 | 2 | 2 | |
| Butyl Ether | 2 | 3 | 1 | |
| Butyl acetate, n- | 1 | 3 | 0 | |
| Butyl acetate, s- | 1 | 3 | 0 | |
| Butylamine | 3 | 3 | 0 | |
| Butylene Oxide | 2 | 3 | 2 | |
| Butyllithium | 3 | 4 | 2 | w |
| Butyraldehyde | 2 | 3 | 2 | |
| Butyric Acid | 3 | 2 | 0 |
| Chemical | Health | Fire | Reactivity | Special |
| Calcium Carbide | 1 | 3 | 2 | w |
| Calcium Chlorate | 1 | 0 | 1 | __ ox |
| Calcium Cyanide, solid | 3 | 0 | 1 | |
| Calcium Hypochlorite | 1 | 0 | 2 | ox |
| Calcium Oxide | 1 | 0 | 1 | |
| Calcium, metal | 1 | 1 | 2 | w |
| Carbon Disulfide | 2 | 4 | 0 | |
| Carbon Monixide | 3 | 4 | 0 | |
| Carbon Tetrachloride | 3 | 0 | 0 | |
| Chlorine | 3 | 0 | 0 | ox |
| Chlorine Trifluoride | 4 | 0 | 3 | |
| Chloroacetic Acid | 3 | 1 | 0 | |
| Chloroacetonitrile | 3 | 2 | 0 | |
| Chloroacetyl Chloride | 3 | 0 | 1 | |
| Chlorobenzene | 2 | 3 | 0 | |
| Chlorodiethyl Silane | 3 | 3 | 1 | |
| Chloroform | 2 | 0 | 0 | |
| Chlorophenols, liquid | 3 | 2 | 0 | |
| Chlorophenols, solids | 3 | 1 | 0 | |
| Chloropicrin, liquid | 4 | 0 | 3 | |
| Chlorosilanes | 3 | 3 | 2 | w |
| Chlorosulfonic Acid | 3 | 0 | 2 | |
| Chromic Acid, solid | 3 | 0 | 1 | ox |
| Chromic chloride | 3 | 0 | 0 | |
| Chromyl Chloride | 2 | 0 | 2 | w |
| Cresol(o-, m-, p-) | 3 | 2 | 0 | |
| Crotonaldehyde | 3 | 3 | 2 | |
| Cumene | 2 | 3 | 1 | |
| Cyanoacetic Acid | 3 | 1 | 0 | |
| Cyanogen Bromide | 3 | 0 | 1 | |
| Cyanogen Gas | 4 | 4 | 2 | |
| Cyclohexane | 1 | 3 | 0 | |
| Cyclohexanol | 1 | 2 | 0 | |
| Cyclohexanone | 1 | 2 | 0 | |
| Cyclohexylamine | 2 | 3 | 0 |
| Chemical | Health | Fire | Reactivity | Special |
| Decaborane | 3 | 2 | 1 | |
| Diamylamine | 3 | 2 | 0 | |
| Diborane | 3 | 4 | 3 | w |
| Dibutylamine | 3 | 2 | 0 | |
| Dichloroacetyl Chloride | 3 | 2 | 2 | w |
| Dichloroanilines | 3 | 1 | 0 | |
| Dichlorobenzene | 2 | 2 | 0 | |
| Dichlorobutane, 1, 4- | 2 | 2 | 0 | |
| Dichlorodimethylether | 3 | 3 | 1 | |
| Dichloroethyl Ether, 2, 2- | 3 | 2 | 1 | |
| Dichloroethylene | 2 | 3 | 2 | |
| Dichloropropene, 1, 3- | 3 | 3 | 0 | |
| Dichlorosilane | 3 | 4 | 2 | w |
| Diesel fuel (No. 1, 2 and 4) | 0 | 2 | 0 | |
| Diethyl Sulfate | 3 | 1 | 1 | |
| Diethyl Telluride | 2 | 4 | 3 | w |
| Diethylaluminum Chloride | 3 | 4 | 3 | w |
| Diethylamine | 3 | 3 | 0 | |
| Diethylenetriamine | 3 | 1 | 0 | |
| Diethylzinc | 1 | 4 | 3 | w |
| Diisopropyl Ether | 2 | 3 | 1 | |
| Diisopropylamine | 3 | 3 | 0 | |
| Diketene, inhibited | 3 | 2 | 2 | |
| Dimethyl Ether | 2 | 4 | 1 | |
| Dimethyl Sulfate | 4 | 2 | 0 | |
| Dimeethyl Sulfide | 2 | 4 | 0 | |
| Dimethylamine | 3 | 4 | 0 | |
| Dimethylhydrazine | 3 | 3 | 1 | |
| Dinitroaniline, 2, 4- | 3 | 1 | 3 | |
| Dinitrobenzene, 1, 2- | 3 | 1 | 4 | |
| Dinitrochlorobenzene | 3 | 1 | 4 | |
| Dinitrotoluene | 3 | 1 | 3 | |
| Dioxane, 1, 4- | 2 | 3 | 1 | |
| Divinylbenzene | 2 | 2 | 2 |
| Chemical | Health | Fire | Reactivity | Special |
| Epichlorohydrin | 3 | 3 | 2 | |
| Ethyl Acrylate, inhibited | 2 | 3 | 2 | |
| Ethyl Chloride | 2 | 4 | 0 | |
| Ethyl Chloroformate | 3 | 3 | 1 | |
| Ethyl Ether | 2 | 4 | 1 | |
| Ethyl Methyl Ether | 2 | 4 | 1 | |
| Ethyl Nitrite | 2 | 4 | 4 | |
| Ethyl Trichlorosilane | 3 | 3 | 2 | w |
| Ethyl acetate | 1 | 3 | 0 | |
| Ethylaniline, N- | 3 | 2 | 0 | |
| Eathylbenzene | 2 | 3 | 0 | |
| Ethylene | 1 | 4 | 2 | |
| Ethylene Chlorohydrin | 4 | 2 | 0 | |
| Ethylene Cyanohydrin | 2 | 1 | 2 | |
| Ethylene Dibromide | 3 | 0 | 0 | |
| Ethylene Dichloride | 2 | 3 | 0 | |
| Ethylene Oxide | 2 | 4 | 3 | |
| Ethylenediamine | 3 | 3 | 0 | |
| Ethyleneimine, inhibited | 3 | 3 | 3 | |
| Ethylhexyl Acrylate, 2- | 2 | 2 | 2 |
| Chemical | Health | Fire | Reactivity | Special |
| Fluoboric Acid | 3 | 3 | 0 | |
| Fluorine | 3 | 0 | 4 | w |
| Formaldehyde, anhydrous | 3 | 4 | 0 | |
| Formaldehyde, aqueous | 3 | 2 | 0 | |
| Formic Acid | 3 | 2 | 0 | |
| Fuel oil (Nos. 1-6) | 0 | 2 | 0 | |
| Furfural | 2 | 2 | 0 |
| Chemical | Health | Fire | Reactivity | Special |
| Gallium Arsenide | 3 | 1 | 2 | w |
| Gallium Phosphide | 3 | 3 | 2 | w |
| Gallium Trichloride | 3 | 0 | 1 | |
| Gasoline | 1 | 3 | 0 | |
| Germane | 4 | 4 | 3 |
| Chemical | Health | Fire | Reactivity | Special |
| Heptane, n- | 1 | 3 | 0 | |
| Hexamethyldisilazane | 3 | 2 | 1 | |
| Hexane, n- | 1 | 3 | 0 | |
| Hexanol, n- | 1 | 2 | 0 | |
| Hydrazine, anhydrous | 3 | 3 | 3 | |
| Hydroidic Acid | 3 | 0 | 0 | |
| Hydrobromic Acid | 3 | 0 | 0 | |
| Hydrogen Chloride | 3 | 0 | 0 | |
| Hydrogen Cyanide | 4 | 4 | 2 | |
| Hydrogen Fluoride | 4 | 0 | 1 | |
| Hydrogen Peroxide (35-52%) | 2 | 0 | 1 | ox |
| Hydrogen Peroxide (>52%) | 2 | 0 | 3 | ox |
| Hydrogen Sulfide | 3 | 4 | 0 | |
| Hydrogen, gas | 0 | 4 | 0 | |
| Hydrogen, liquefied | 3 | 4 | 0 | |
| Hydroxylamine | 2 | 0 | 3 |
| Chemical | Health | Fire | Reactivity | Special |
| Isobutyl acetate | 1 | 3 | 0 | |
| Isophorone | 2 | 2 | 0 | |
| Isoprene | 2 | 4 | 2 | |
| Isopropanol | 1 | 3 | 0 | |
| Isopropyl Formate | 2 | 3 | 0 | |
| Isopropylamine | 3 | 4 | 0 |
| Chemical | Health | Fire | Reactivity | Special |
| Kerosene | 0 | 2 | 0 | |
| Lead Arsenates | 2 | 0 | 0 | |
| Lithium Alunimum Hydride | 3 | 2 | 2 | w |
| Lithium Hydride | 3 | 2 | 2 | w |
| Lithium Metal | 3 | 2 | 2 | w |
| Chemical | Health | Fire | Reactivity | Special |
| Magnesium (+alloys) | 0 | 1 | 1 | |
| Maleic Anhydride | 3 | 1 | 1 | |
| Mercuric Cyanide | 3 | 0 | 0 | |
| Mesityl Oxide | 3 | 3 | 1 | |
| Methacrylic Acid, inhibited | 3 | 2 | 2 | |
| Methyl Acrylate, inhibited | 2 | 3 | 2 | |
| Methyl Bromide | 3 | 1 | 0 | |
| Methyl Butyraldehyde, 2- | 2 | 3 | 0 | |
| Methyl Chloride | 2 | 4 | 0 | |
| Methyl Chloromethyl Ether | 3 | 3 | 2 | |
| Methyl cyclopenate | 2 | 3 | 0 | |
| Methyl Formate | 2 | 4 | 0 | |
| Methyl Hydrazine | 3 | 3 | 2 | |
| Methyl Isocyanate | 4 | 3 | 2 | |
| Methyl methacrylate | 2 | 3 | 2 | |
| Methyl Vinyl Ketone, inhibited | 3 | 3 | 2 | |
| Methyl acetate | 1 | 3 | 0 | |
| Methyl alcohol | 1 | 3 | 0 | |
| Methyl ethyl keetone | 1 | 3 | 0 | |
| Methyl mercaptan | 2 | 4 | 0 | |
| Methyl-1-butene, 3- | 2 | 4 | 0 | |
| Methyl-2-butanol, 2- | 1 | 3 | 0 | |
| Methyl-2-butanol, 3- | 1 | 3 | 0 | |
| Methyl-2-propanol, 2- | 1 | 3 | 0 | |
| Methyl-5-Pyridine, 2- | 2 | 2 | 0 | |
| Methylamine, anhydrous | 3 | 4 | 0 | |
| Methyldichlorosilane | 3 | 3 | 2 | w |
| Methylene Chloride | 2 | 1 | 0 | |
| Methyltrichlorosilane | 3 | 3 | 2 | w |
| Monochloro-s-Triazinetrione Acid | 3 | 0 | 2 | |
| Monoethanolamine | 2 | 2 | 0 | |
| Monoethylamine | 3 | 4 | 0 | |
| Morpholine | 2 | 3 | 0 |
| Chemical | Health | Fire | Reactivity | Special |
| Napthalene | 2 | 2 | 0 | |
| Natural Gas, gas | 0 | 4 | 0 | |
| Natural Gas, liquid | 3 | 4 | 1 | |
| Nickel Carbonyl | 4 | 3 | 3 | |
| Nickel Catalyst, dry | 2 | 3 | 3 | |
| Nitric Acid, >40% | 3 | 0 | 0 | ox |
| Nitric Acid, fuming | 3 | 0 | 1 | ox |
| Nitroaniline, p- | 3 | 1 | 2 | |
| Nitrobenzene, liquid | 3 | 2 | 1 | |
| Nitrocellulose, alcohol wet | 2 | 3 | 2 | |
| Nitrocellulose, colloided | 2 | 3 | 0 | |
| Nitrocellulose, water wet | 1 | 2 | 2 | |
| Nitrochlorobenzene | 3 | 1 | 0 | |
| Nitroethane | 1 | 3 | 3 | |
| Nitrogen oxides | 3 | 0 | 0 | ox |
| Nitrogen, refrig liquid | 3 | 0 | 0 | |
| Nitromethane | 1 | 3 | 4 | |
| Nitrophenol, p- | 3 | 1 | 2 | |
| Nitropropane | 1 | 3 | 2 | |
| Nitrotoluene, o-, m-, p- | 3 | 1 | 1 |
| Chemical | Health | Fire | Reactivity | Special |
| Octane | 0 | 3 | 0 | |
| Octyl alcohol | 1 | 2 | 0 | |
| Oxalic Acid, dihydrate | 2 | 1 | 0 | |
| Oxygen, gas | 0 | 0 | 0 | ox |
| Oxygen, liquid | 3 | 0 | 0 | ox |
| Chemical | Health | Fire | Reactivity | Special |
| Paraformaldehyde | 2 | 1 | 0 | |
| Paraldehyde | 2 | 3 | 1 | |
| Pentaborane | 4 | 4 | 2 | |
| Pentachlorophenol | 3 | 0 | 0 | |
| Pentane | 1 | 4 | 0 | |
| Pentanol, 1- | 1 | 3 | 0 | |
| Pentanol, 2- | 3 | 2 | 0 | |
| Pentanol, 3- | 1 | 2 | 0 | |
| Pentenel, 1- | 1 | 4 | 0 | |
| Peracetic Acid >40% | 3 | 2 | 4 | ox |
| Perchloric Acid (>50%>72%) | 3 | 0 | 3 | ox |
| Petroleum crude | 1 | 3 | 0 | |
| Petroleum ether | 1 | 4 | 0 | |
| Phenol | 3 | 2 | 0 | |
| Phenylethanolamine | 2 | 1 | 0 | |
| Phenylmercuric Acetate, dry | 3 | 1 | 0 | |
| Phenylmercuric Acetate, organic | 3 | 2 | 0 | |
| Phosphorus Oxychloride | 3 | 0 | 2 | w |
| Phosphorus Pentachloride | 3 | 0 | 2 | w |
| Phosphorus Pentasulfide | 2 | 1 | 2 | w |
| Phosphorus Tribromide | 3 | 0 | 2 | w |
| Phosphorus Trichloride | 3 | 0 | 2 | w |
| Phosphorus, amorphous, red | 1 | 1 | 1 | |
| Phthalic Anhydride | 2 | 1 | 0 | |
| Picric Acid, 10% water | 3 | 4 | 4 | |
| Polychlorinated Biphenyls | 2 | 1 | 0 | |
| Potassium Chlorate | 1 | 0 | 1 | ox |
| Potassium Cyanide, solid | 3 | 0 | 0 | |
| Potassium Dichloro-s-Triazinetrione | 3 | 0 | 2 | ox |
| Potassium Hydroxide | 3 | 0 | 1 | |
| Potassium Perchlorate | 1 | 0 | 1 | ox |
| Potassium Peroxide | 3 | 0 | 1 | ox |
| Potassium Sulfide | 3 | 1 | 0 | |
| Potassium, metal | 3 | 3 | 2 | w |
| Propargyl Alcohol | 3 | 3 | 3 | |
| Propionaldehyde | 2 | 3 | 2 | |
| Propionic Acid | 2 | 2 | 0 | |
| Propionic Anhydride | 2 | 2 | 1 | |
| Propyl Nitrate | 2 | 3 | 3 | ox |
| Propyl acetate | 1 | 3 | 0 | |
| Propylamine, n- | 3 | 3 | 0 | |
| Propylene | 1 | 4 | 1 | |
| Propylene Dichloride | 2 | 3 | 0 | |
| Propylene Oxide | 4 | 2 | 2 | |
| Propyltrichlorosilane | 3 | 3 | 1 | |
| Pyridine | 2 | 3 | 0 | |
| Pyroxylin Plastic | 2 | 3 | 2 |
| Chemical | Health | Fire | Reactivity | Special |
| Silane | 2 | 4 | 3 | |
| Silane, (4-Aminobutyl)-Diethoxymethyl | 3 | 2 | 1 | |
| Silicon Tetrachloride | 3 | 0 | 2 | w |
| Silicon Tetrafluoride | 3 | 0 | 2 | w |
| Sodium Chlorate | 1 | 0 | 1 | ox |
| Sodium Chlorite | 1 | 1 | 1 | ox |
| Sodium Cyanide | 3 | 0 | 0 | |
| Sodium Dichloro-s-Triazinetrione and Dihydra | 3 | 3 | 2 | |
| Sodium Fluoride | 2 | 0 | 0 | |
| Sodium Hydride | 3 | 3 | 2 | w |
| Sodium Hydrosulfite | 3 | 1 | 2 | |
| Sodium Hydroxide | 3 | 0 | 1 | |
| Sodium Perchlorate | 2 | 0 | 1 | ox |
| Sodium Peroxide | 3 | 0 | 1 | ox |
| Sodium Potassium Alloys | 3 | 3 | 2 | w |
| Sodium Sulfide, anhydrous | 3 | 1 | 1 | |
| Sodium, metal | 3 | 3 | 2 | w |
| Stibine | 3 | 4 | 2 | |
| Styrene monomer, inhibited | 2 | 3 | 2 | |
| Sulfur Dioxide | 3 | 0 | 0 | |
| Sulfur Monochloride | 2 | 1 | 1 | |
| Sulfur, dry | 1 | 1 | 0 | |
| Sulfur, molten | 2 | 1 | 0 | |
| Sulfuric Acid | 3 | 0 | 2 | w |
| Sulfuryl Chloride | 3 | 0 | 1 |
| Chemical | Health | Fire | Reactivity | Special |
| Tetrachloroethylene | 2 | 0 | 0 | |
| Tetrafluorethylene (monomer, inhibited) | 2 | 4 | 3 | |
| Tetrahydrofuran | 2 | 3 | 1 | |
| Tetrahydronaphthalene | 1 | 2 | 0 | |
| Tetramethoxysilane | 3 | 3 | 1 | |
| Thionyl Chloride | 3 | 0 | 2 | w |
| Tin Tetrachloride, anhydrous | 3 | 0 | 1 | |
| Titanium Tetrachloride | 3 | 0 | 2 | w |
| Toluene | 2 | 3 | 0 | |
| Toluene, 2, 4-Disocyanate | 3 | 1 | 2 | |
| Toluidine, o- | 3 | 2 | 0 | |
| Toluidine, p- | 3 | 2 | 0 | |
| Tributylamine | 3 | 2 | 0 | |
| Trichloroethane, 1, 1, 2- | 3 | 1 | 0 | |
| Trichloroethylene | 2 | 2 | 0 | |
| Trichloroisocyanuric Acid | 3 | 0 | 2 | ox |
| Trichlorosilane | 3 | 4 | 2 | w |
| Triethylaluminum | 3 | 4 | 3 | w |
| Triethylamine | 2 | 3 | 0 | |
| Triisobutylaluminum | 3 | 4 | 3 | w |
| Trimethoxxysilane | 3 | 3 | 2 | |
| Trimethylamine | 3 | 4 | 0 | |
| Tripentylamine | 2 | 1 | 0 | |
| Turpentine | 1 | 3 | 0 |
| Chemical | Health | Fire | Reactivity | Special |
| Vanadium Tetrachloride | 3 | 0 | 2 | w |
| Vinyl Acetate | 2 | 3 | 2 | |
| Vinyl Acetylene | 2 | 4 | 3 | |
| Vinyl Chloride | 2 | 4 | 2 | |
| Vinyl Ether | 2 | 3 | 2 | |
| Vinylidene Chloride, inhibited | 2 | 4 | 2 | |
| Vinyltoluene | 2 | 2 | 2 | |
| Xylenes, o-, m-, p- | 2 | 3 | 0 | |
| Zylidine, o- | 3 | 1 | 0 | |
| Zinc Chlorate | 1 | 0 | 1 | ox |
| Zinc Phosphide | 3 | 3 | 1 | |
| Zirconium Tetrachloride | 3 | 0 | 2 | w |
|
NOTE: NFPA and other labeling codes are NOT required by OSHA. OSHA has said "...OSHA does not endorse specific services or products. It would, therefore, be inappropriate for OSHA to require a particular labeling system's code on the material safety data sheet." (see official OSHA interpretations). OSHA does have specific labeling requirements that must be fulfilled, but there is no specified format or code system required. |
Labels
HEALTH HAZARD: BLUE
 |
0 | Material which on exposure under fire conditions would offer no hazard beyond that of ordinary combustible material. | Example: peanut oil |
| 1 | Material which on exposure would cause irritation but only minor residual injury even if no treatment is given. | Example: turpentine |
|
| 2 | Material that on intense or continued but not chronic exposure could cause temporary incapacitation or possible residual injury. | Example: ammonia gas |
|
| 3 | Material that on short exposure could cause serious temporary or residual injury. | Example: chlorine gas |
|
| 4 | Material that on very short exposure could cause death or major residual injury. | Example: hydrogen cyanide |
FLAMMABILITY HAZARD: RED
 |
0 | Material will not burn. | Example: water |
| 1 | Material must be pre-heated before ignition can occur. Flash Point At or Above 200oF (93.4oC) |
Example: corn oil |
|
| 2 | Material must be moderately heated or exposed to relatively high ambient temperature before ignition can occur. Flash Point At or Above 100oF (37.8oC) - Below 200oF (93.4oC) |
Example: diesel fuel oil |
|
| 3 | Liquids and solids that can be ignited under almost all ambient temperature conditions. Flash Point At or Above 73oF (22.8oC) - Below 100oF (37.8oC) Boiling Point At or Above 100oF (37.8oC) |
Example: gasoline |
|
| 4 | Materials that will rapidly or completely vaporize at atmospheric pressure and normal ambient temperature, or that are readily dispersed in air and that will burn readily. Flash Point Below 73oF (22.8oC) Boiling Point Below 100oF (37.8oC) |
Example: propane gas |
REACTIVITY HAZARD: YELLOW
 |
0 | Material that in itself is normally stable, even under fire exposure conditions, and is not reactive with water. | Example: liquid nitrogen |
| 1 | Material that in itself is normally stable, but which can become unstable at elevated temperatures and pressures. | Example: phosphorus (red or white) |
|
| 2 | Material that readily undergoes violent chemical change at elevated temperatures and pressures or which reacts violently with water or which may form explosive mixtures with water. | Example: calcium metal |
|
| 3 | Material that in itself is capable of detonation or explosive decomposition or reaction but requires a strong initiating source or which must be heated under confinement before initiation or which reacts explosively with water. | Example: fluorine gas |
|
| 4 | Material that in itself is readily capable of detonation or of explosive decomposition or reaction at normal temperatures and pressures. | Example: trinitrotoluene (TNT) |
SPECIAL NOTES: WHITE
| The fourth, white, field of the hazard signal can have variable content, depending on who prepared the signal. The 1990 edition of the National Fire Codes (section 704, chapter 5) specifies only "TWO" NFPA 704 approved symbols. Additional symbols are commonly included. The field may also be left blank if no special hazards are present. | |||
 |
OX | Material possesses oxidizing properties. A chemical which can greatly increase the rate of combustion/fire. | Example: ammonium nitrate (fertilizer used in Oklahoma City bomb) |
| Unusual reactivity with water. This indicates a potential hazard using water to fight a fire involving this material.(i.e. don't put water on it) | Example: magnesium metal |
||
| Other symbols, abbreviations, and words that some organizations use in the white Special Hazards section are shown below. These uses are not compliant with NFPA 704, but we present them here in case you see them on an MSDS or container label: | ||
 |
ACID | This indicates that the material is an acid, a corrosive material that has a pH lower than 7.0 |
| ALK | This denotes an alkaline material, also called a base. These caustic materials have a pH greater than 7.0 | |
| COR | This denotes a material that is corrosive (it could be either an acid or a base). | |
 |
The international symbol for radioactivity is used to denote radioactive hazards; radioactive materials are extremely hazardous when inhaled. | |
 |
This is a another symbol used for corrosive. | |
 |
The skull and crossbones is used to denote a poison or highly toxic material. | |
 |
Indicates an explosive material. This symbol is somewhat redundant because explosives are easily recognized by their Instability Rating. | |